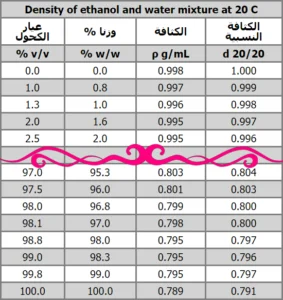
The ethanol density table for the ethanol-water mixture at 20 C. It contains Percentages by volume, % by weight, ethanol density, and relative densities.
-
Dilution of ethanol using ethanol density table
- 1- Preparation of a weight concentration of pure absolute ethanol
- 2- Adding water to an amount of pure absolute ethanol to reach a specified concentration
- 3- Prepare a volumetric solution of pure absolute ethanol
- 4- Preparation of a specific w/w concentration using 96% v/v ethanol
- 5- diluting a specific weight of 96% v/v ethanol to reach a concentration of 70% w/w
| Density of ethanol and water mixture at 20 C | |||
|---|---|---|---|
| عيار الكحول | % وزنا | الكثافة | الكثافة النسبية |
| % v/v | % w/w | ρ g/mL | d 20/20 |
| 0.0 | 0.0 | 0.998 | 1.000 |
| 1.0 | 0.8 | 0.997 | 0.999 |
| 1.3 | 1.0 | 0.996 | 0.998 |
| 2.0 | 1.6 | 0.995 | 0.997 |
| 2.5 | 2.0 | 0.995 | 0.996 |
| 3.0 | 2.4 | 0.994 | 0.996 |
| 3.8 | 3.0 | 0.993 | 0.995 |
| 4.0 | 3.2 | 0.992 | 0.994 |
| 5.0 | 4.0 | 0.991 | 0.993 |
| 6.0 | 4.8 | 0.990 | 0.992 |
| 6.3 | 5.0 | 0.989 | 0.991 |
| 7.0 | 5.6 | 0.988 | 0.990 |
| 7.5 | 6.0 | 0.988 | 0.990 |
| 8.0 | 6.4 | 0.987 | 0.989 |
| 8.7 | 7.0 | 0.986 | 0.988 |
| 9.0 | 7.2 | 0.986 | 0.988 |
| 10.0 | 8.0 | 0.985 | 0.987 |
| 11.0 | 8.8 | 0.984 | 0.986 |
| 11.2 | 9.0 | 0.983 | 0.985 |
| 12.0 | 9.6 | 0.982 | 0.984 |
| 12.4 | 10.0 | 0.982 | 0.984 |
| 13.0 | 10.5 | 0.981 | 0.983 |
| 13.7 | 11.0 | 0.980 | 0.982 |
| 14.0 | 11.3 | 0.980 | 0.982 |
| 14.9 | 12.0 | 0.979 | 0.981 |
| 15.0 | 12.1 | 0.979 | 0.981 |
| 16.0 | 12.9 | 0.978 | 0.980 |
| 16.1 | 13.0 | 0.978 | 0.980 |
| 17.0 | 13.7 | 0.977 | 0.979 |
| 17.3 | 14.0 | 0.976 | 0.978 |
| 18.0 | 14.6 | 0.976 | 0.978 |
| 18.5 | 15.0 | 0.975 | 0.977 |
| 19.0 | 15.4 | 0.975 | 0.977 |
| 19.7 | 16.0 | 0.974 | 0.976 |
| 20.0 | 16.2 | 0.974 | 0.976 |
| 20.9 | 17.0 | 0.973 | 0.974 |
| 21.0 | 17.1 | 0.972 | 0.974 |
| 22.0 | 17.9 | 0.971 | 0.973 |
| 22.1 | 18.0 | 0.971 | 0.973 |
| 23.0 | 18.7 | 0.970 | 0.972 |
| 23.3 | 19.0 | 0.970 | 0.972 |
| 24.0 | 19.6 | 0.969 | 0.971 |
| 24.5 | 20.0 | 0.969 | 0.970 |
| 25.0 | 20.4 | 0.968 | 0.970 |
| 25.7 | 21.0 | 0.967 | 0.969 |
| 26.0 | 21.2 | 0.967 | 0.969 |
| 26.9 | 22.0 | 0.966 | 0.968 |
| 27.0 | 22.1 | 0.966 | 0.968 |
| 28.0 | 22.9 | 0.965 | 0.967 |
| 28.1 | 23.0 | 0.965 | 0.966 |
| 29.0 | 23.8 | 0.963 | 0.965 |
| 29.3 | 24.0 | 0.963 | 0.965 |
| 30.0 | 24.6 | 0.962 | 0.964 |
| 30.5 | 25.0 | 0.962 | 0.963 |
| 31.0 | 25.5 | 0.961 | 0.963 |
| 31.6 | 26.0 | 0.960 | 0.962 |
| 32.0 | 26.3 | 0.960 | 0.962 |
| 32.8 | 27.0 | 0.959 | 0.960 |
| 33.0 | 27.2 | 0.958 | 0.960 |
| 34.0 | 28.0 | 0.957 | 0.959 |
| 35.0 | 28.9 | 0.956 | 0.958 |
| 35.1 | 29.0 | 0.955 | 0.957 |
| 36.0 | 29.8 | 0.954 | 0.956 |
| 36.3 | 30.0 | 0.954 | 0.956 |
| 37.0 | 30.6 | 0.953 | 0.955 |
| 37.4 | 31.0 | 0.952 | 0.954 |
| 38.0 | 31.5 | 0.951 | 0.953 |
| 38.5 | 32.0 | 0.950 | 0.952 |
| 39.0 | 32.4 | 0.950 | 0.952 |
| 39.7 | 33.0 | 0.949 | 0.950 |
| 40.0 | 33.3 | 0.948 | 0.950 |
| 40.8 | 34.0 | 0.947 | 0.948 |
| 41.0 | 34.2 | 0.946 | 0.948 |
| 41.9 | 35.0 | 0.945 | 0.947 |
| 42.0 | 35.1 | 0.945 | 0.947 |
| 43.0 | 36.0 | 0.943 | 0.945 |
| 44.0 | 36.9 | 0.941 | 0.943 |
| 44.1 | 37.0 | 0.941 | 0.943 |
| 45.0 | 37.8 | 0.940 | 0.942 |
| 45.2 | 38.0 | 0.939 | 0.941 |
| 46.0 | 38.7 | 0.938 | 0.940 |
| 46.3 | 39.0 | 0.937 | 0.939 |
| 47.0 | 39.6 | 0.936 | 0.938 |
| 47.4 | 40.0 | 0.935 | 0.937 |
| 48.0 | 40.6 | 0.934 | 0.936 |
| 48.5 | 41.0 | 0.933 | 0.935 |
| 49.0 | 41.5 | 0.932 | 0.934 |
| 49.5 | 42.0 | 0.931 | 0.933 |
| 50.0 | 42.4 | 0.930 | 0.932 |
| 50.6 | 43.0 | 0.929 | 0.931 |
| 51.0 | 43.4 | 0.928 | 0.930 |
| 51.7 | 44.0 | 0.927 | 0.929 |
| 52.0 | 44.3 | 0.926 | 0.928 |
| 52.7 | 45.0 | 0.925 | 0.926 |
| 53.0 | 45.3 | 0.924 | 0.926 |
| 53.8 | 46.0 | 0.923 | 0.924 |
| 54.0 | 46.2 | 0.922 | 0.924 |
| 54.8 | 47.0 | 0.920 | 0.922 |
| 55.0 | 47.2 | 0.920 | 0.922 |
| 55.8 | 48.0 | 0.918 | 0.920 |
| 56.0 | 48.2 | 0.918 | 0.920 |
| 56.9 | 49.0 | 0.916 | 0.918 |
| 57.0 | 49.1 | 0.916 | 0.918 |
| 57.9 | 50.0 | 0.914 | 0.915 |
| 58.0 | 50.1 | 0.914 | 0.916 |
| 58.9 | 51.0 | 0.912 | 0.913 |
| 59.0 | 51.1 | 0.911 | 0.913 |
| 59.9 | 52.0 | 0.909 | 0.911 |
| 60.0 | 52.1 | 0.909 | 0.911 |
| 60.9 | 53.0 | 0.907 | 0.909 |
| 61.0 | 53.1 | 0.907 | 0.909 |
| 61.9 | 54.0 | 0.905 | 0.906 |
| 62.0 | 54.1 | 0.905 | 0.907 |
| 62.9 | 55.0 | 0.903 | 0.904 |
| 63.0 | 55.1 | 0.902 | 0.904 |
| 63.9 | 56.0 | 0.900 | 0.902 |
| 64.0 | 56.1 | 0.900 | 0.902 |
| 64.8 | 57.0 | 0.898 | 0.900 |
| 65.0 | 57.1 | 0.898 | 0.900 |
| 65.8 | 58.0 | 0.896 | 0.897 |
| 66.0 | 58.2 | 0.895 | 0.897 |
| 66.8 | 59.0 | 0.893 | 0.895 |
| 67.0 | 59.2 | 0.893 | 0.895 |
| 67.7 | 60.0 | 0.891 | 0.893 |
| 68.0 | 60.3 | 0.890 | 0.892 |
| 68.7 | 61.0 | 0.889 | 0.890 |
| 69.0 | 61.3 | 0.888 | 0.890 |
| 69.6 | 62.0 | 0.887 | 0.888 |
| 70.0 | 62.4 | 0.886 | 0.888 |
| 70.6 | 63.0 | 0.884 | 0.886 |
| 71.0 | 63.5 | 0.883 | 0.885 |
| 71.5 | 64.0 | 0.882 | 0.883 |
| 72.0 | 64.5 | 0.881 | 0.883 |
| 72.4 | 65.0 | 0.879 | 0.881 |
| 73.0 | 65.6 | 0.878 | 0.880 |
| 73.3 | 66.0 | 0.877 | 0.879 |
| 74.0 | 66.8 | 0.875 | 0.877 |
| 74.3 | 67.0 | 0.875 | 0.876 |
| 75.0 | 67.8 | 0.873 | 0.875 |
| 75.2 | 68.0 | 0.872 | 0.874 |
| 76.0 | 69.0 | 0.870 | 0.872 |
| 76.1 | 69.0 | 0.870 | 0.872 |
| 76.9 | 70.0 | 0.868 | 0.869 |
| 77.0 | 70.1 | 0.867 | 0.869 |
| 77.8 | 71.0 | 0.865 | 0.867 |
| 78.0 | 71.2 | 0.865 | 0.867 |
| 78.7 | 72.0 | 0.863 | 0.864 |
| 79.0 | 72.3 | 0.862 | 0.864 |
| 79.6 | 73.0 | 0.860 | 0.862 |
| 80.0 | 73.5 | 0.859 | 0.861 |
| 80.4 | 74.0 | 0.858 | 0.860 |
| 81.0 | 74.7 | 0.856 | 0.858 |
| 81.3 | 75.0 | 0.856 | 0.857 |
| 82.0 | 75.8 | 0.854 | 0.856 |
| 82.2 | 76.0 | 0.853 | 0.855 |
| 83.0 | 77.0 | 0.851 | 0.852 |
| 83.8 | 78.0 | 0.848 | 0.850 |
| 84.0 | 78.2 | 0.848 | 0.850 |
| 84.7 | 79.0 | 0.846 | 0.847 |
| 85.0 | 79.4 | 0.845 | 0.847 |
| 85.5 | 80.0 | 0.843 | 0.845 |
| 86.0 | 80.6 | 0.842 | 0.844 |
| 86.3 | 81.0 | 0.841 | 0.842 |
| 87.0 | 81.9 | 0.839 | 0.841 |
| 87.1 | 82.0 | 0.838 | 0.840 |
| 87.9 | 83.0 | 0.836 | 0.837 |
| 88.0 | 83.1 | 0.836 | 0.838 |
| 88.7 | 84.0 | 0.833 | 0.835 |
| 89.0 | 84.3 | 0.833 | 0.834 |
| 89.5 | 85.0 | 0.831 | 0.832 |
| 90.0 | 85.7 | 0.829 | 0.830 |
| 90.3 | 86.0 | 0.828 | 0.830 |
| 91.0 | 87.0 | 0.826 | 0.827 |
| 91.8 | 88.0 | 0.823 | 0.825 |
| 92.0 | 88.3 | 0.822 | 0.823 |
| 92.5 | 89.0 | 0.821 | 0.822 |
| 93.0 | 89.6 | 0.819 | 0.820 |
| 93.3 | 90.0 | 0.818 | 0.819 |
| 94.0 | 91.0 | 0.815 | 0.817 |
| 94.7 | 92.0 | 0.813 | 0.814 |
| 95.0 | 92.5 | 0.811 | 0.812 |
| 95.1 | 92.6 | 0.811 | 0.812 |
| 95.4 | 93.0 | 0.810 | 0.811 |
| 96.0 | 93.9 | 0.807 | 0.808 |
| 96.1 | 94.0 | 0.807 | 0.809 |
| 96.8 | 95.0 | 0.804 | 0.806 |
| 96.9 | 95.1 | 0.804 | 0.805 |
| 97.0 | 95.3 | 0.803 | 0.804 |
| 97.5 | 96.0 | 0.801 | 0.803 |
| 98.0 | 96.8 | 0.799 | 0.800 |
| 98.1 | 97.0 | 0.798 | 0.800 |
| 98.8 | 98.0 | 0.795 | 0.797 |
| 99.0 | 98.3 | 0.795 | 0.796 |
| 99.8 | 99.0 | 0.795 | 0.797 |
| 100.0 | 100.0 | 0.789 | 0.791 |
for more info about the density of ethanol see https://en.wikipedia.org/wiki/Ethanol
Dilution of ethanol using ethanol density table
Ethanol is usually sold in volumetric concentrations, other concentrations may be required to be prepared from it. dilution calculations are not as easy as they appear. thought they are divided into the following sections
1- Preparation of a weight concentration of pure absolute ethanol
To prepare a concentration of 70% by weight, for example, from ethanol, we take 70 grams of pure ethanol with 30 grams of water, and this is the concentration recommended by the World Health Organization for COVID-19.
Amount of alcohol by weight = quantity to be prepared × desired concentration / 100
Amount of water by weight = Amount to be prepared – Amount of alcohol required
Example 1 – 1: preparing 1000 g of ethanol 70% w/w using pure absolute ethanol
Amount of alcohol by weight = 1000 × 70 / 100= 700 grams
Amount of water by weight = 1000 – 700 = 300 grams
2- Adding water to an amount of pure absolute ethanol to reach a specified concentration
If we have a quantity of absolute alcohol, let it be 1000 grams, and we want to prepare 70% w/w alcohol from it, how much water is required to be added
If the amount of alcohol available is by volume, it is converted to weight by multiplying by density
Weight of available pure alcohol = volume of available pure alcohol × density of pure alcohol
The density of pure alcohol: ρ = 0.789 g/mL
Determine the total weight produced
Total weight = weight of pure alcohol × 100 / required concentration
Determine the weight of the water
Weight of water = total weight – the weight of alcohol
Example 2 – 1: adding water to a 1000ml of pure absolute ethanol to reach a specified 70% w/w
Available alcohol weight = Volume of alchohol × alchohol density
Available alcohol weight = 1000 × 0.789 = 789 grams
Total weight = weight of pure alcohol × 100 / required concentration
Total weight = 789 × 100 / 70 = 1127 grams
Water weight = 1127 – 789 = 338 grams
Example 2- 2- dilution of 160 kg of pure alcohol
Available alcohol weight = 160 kg
Total weight = 160 × 100 / 70 = 228 kilograms
Water weight = 228 – 160 = 68 kg
3- Prepare a volumetric solution of pure absolute ethanol
For the preparation of ethanol a concentration of 76.9% v/v from pure ethanol
from the ethanol density table, we seek the weight percentage that meets the required volumetric percentage.
From the table 76.9% v/v is equal to 70% w/w
After this step, we go ahead as example 1
4- Preparation of a specific w/w concentration using 96% v/v ethanol
Ethanol is sold in the market in volumetric concentration, and for the purpose of sterilization, ethanol at a concentration of 70% by weight is required
The first step is to know the weight concentration corresponding to the purchased ethanol. Then we look at the ethanol density table
We note that 96% v/v (by volume) corresponds to 93.9% w/w (by weight)
Weight of commercial alcohol required =
quantity to be prepared (g) × concentration of alcohol to be prepared ÷ concentration of commercial alcohol
Weight of water required = Amount to be prepared – Weight of commercial alcohol required
Example 4- 1: preparing 1000 g of 70% w/w alcohol from ethanol 96% v/v.
From the ethanol density table, we find that 96% by volume of ethanol is equivalent to 93.9% by weight
Weight of commercial alcohol required = 1000 × 70 ÷ 93.9 = 745 g
Required water weight = 1000 – 745 = 255 grams
Example 4- 2: preparing 1000 ml of 70% w/w alcohol from ethanol 96% v/v.
From the ethanol density table, we find that 96% by volume of ethanol is equivalent to 93.9% by weight
Weight of required = volume x denisty
Density of 70% w/w alchol form the ethanol density table is: ρ = 0.868 g/mL
Weight required = Volume required × density = 1000 × 0.868 = 868 g.
Weight of commercial alcohol required = 868 × 70 ÷ 93.9 = 647 g
Required water weight = 868 – 647= 221 grams
5- diluting a specific weight of 96% v/v ethanol to reach a concentration of 70% w/w
- If the available quantity is in kilos, we will take it as is
- If the available quantity is in liters, we do the following
- We take the density of alcohol available from the table of the density of ethanol
- We multiply the volume of ethanol by its density to determine its weight
- Weight of ethanol = volume of ethanol × density of available ethanol
from the ethanol density table we found that 96% v/v = 93.9% w/w by weight
Total weight produced =
Weight of available ethanol × required ethanol concentration % w/w
÷ commercial alcohol concentration % w/w
Weight of water required = Total weight produced – Weight of commercial alcohol required
Example 5- 1- diluting 1000 ml of ethanol 96% v/v to reach a concentration of 70% w/w
Determining the weight of alcohol available:
* From the table of the density of ethanol, we find that the density of ethanol 96% is 0.807
* Available weight of ethanol is 1000 × 0.807 = 807 g
The weight concentration of ethanol available from the ethanol density table, we find that 96% by volume of ethanol is equivalent to 93.9% by weight
Weight of the resulting achohol = 807 × 93.9 ÷ 70 = 1082 grams
Required water weight = 1082 – 807 = 275 grams
Example 5- 2- A second practical application: extending 160 kg of 96% v/v alcohol to reach 70% w/w ethanol
Determining the weight of alcohol available:
* The available weight of ethanol is 160 kg given by weight, so there is no need to multiply it with anything
Total weight of the product = 160 × 93.9 ÷ 70 = 214 kg
Weight of water required = 214 – 160 = 54 kg
Example 5- 3: diluting of 160 kg of alcohol 99% v/v by volume to reach ethanol 75% by weight
from the ethanol density table 99% by volume of ethanol is equivalent to 98.3% by weight
Total weight of the product = 160 × 98.3 ÷ 75 = 210 kg
Weight of water required = 210 – 160 = 50 kg